Jordan Medical Devices Importation Regulations . Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web medical devices sector in jordan: Jordan requires usfda, ce mark, or japanese. The ministry of health (moh). What certification is required to import medical devices to jordan? Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. Web the food and drug administration (jfda), under the ministry of health (moh), regulates all medical devices in jordan. The directorate of controlled mds and cosmetics controls medical devices, including. Web staff of directorate of medical devices and supplies/cosmetics registration depart Web medical device regulations and classification in jordan.
from vyomusconsulting.com
What certification is required to import medical devices to jordan? Web the food and drug administration (jfda), under the ministry of health (moh), regulates all medical devices in jordan. Jordan requires usfda, ce mark, or japanese. Web staff of directorate of medical devices and supplies/cosmetics registration depart Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web medical device regulations and classification in jordan. The directorate of controlled mds and cosmetics controls medical devices, including. Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. Web medical devices sector in jordan: The ministry of health (moh).
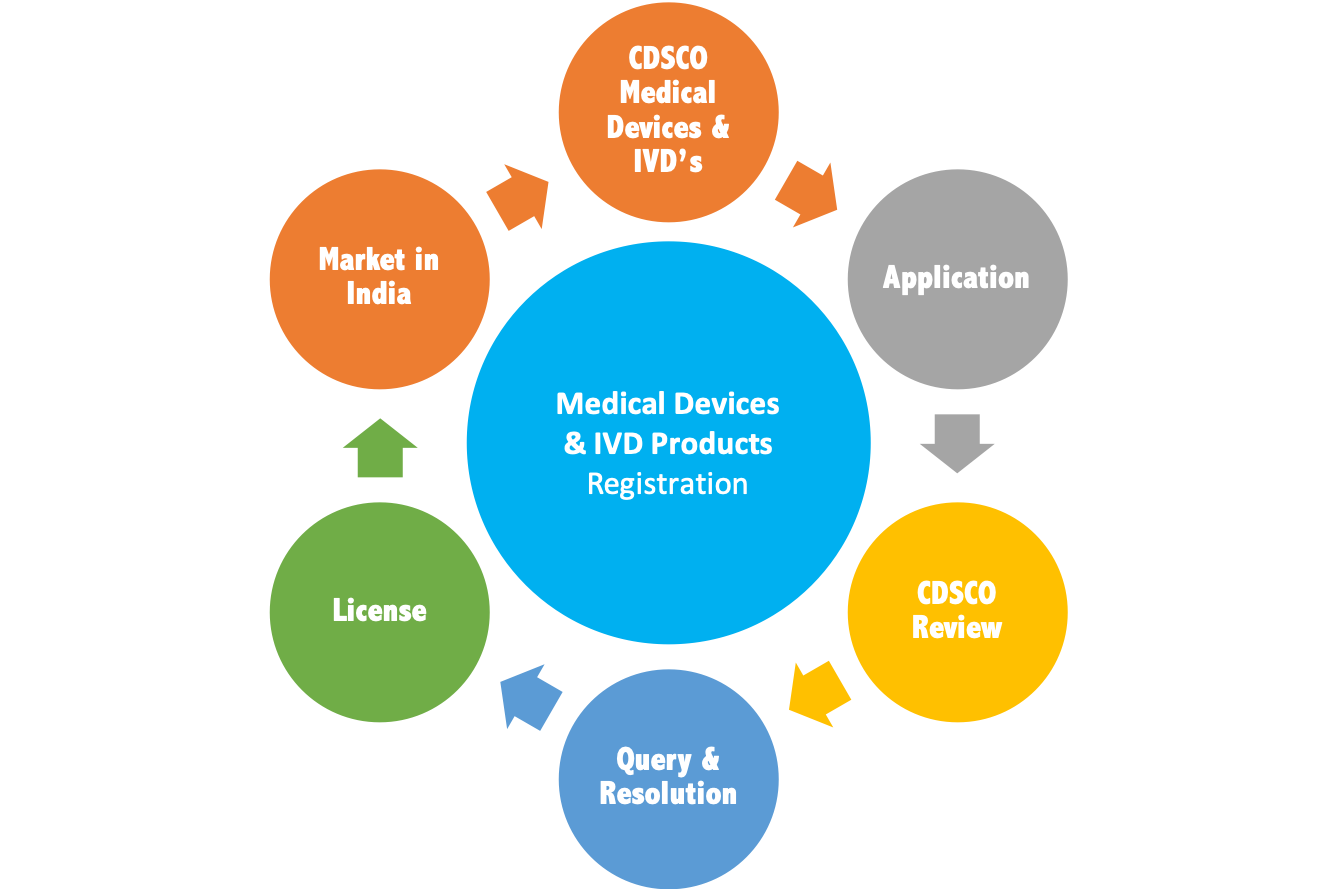
MedicalDevice & IVD Registration Process Overview
Jordan Medical Devices Importation Regulations The ministry of health (moh). The directorate of controlled mds and cosmetics controls medical devices, including. Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. The ministry of health (moh). Web medical device regulations and classification in jordan. Jordan requires usfda, ce mark, or japanese. Web the food and drug administration (jfda), under the ministry of health (moh), regulates all medical devices in jordan. Web staff of directorate of medical devices and supplies/cosmetics registration depart What certification is required to import medical devices to jordan? Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. Web medical devices sector in jordan:
From www.complianceonline.com
Importing Medical Devices 5 Key Factors to Ensure Regulatory Compliance Jordan Medical Devices Importation Regulations The ministry of health (moh). Web staff of directorate of medical devices and supplies/cosmetics registration depart Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. The directorate of controlled mds and. Jordan Medical Devices Importation Regulations.
From www.kff.org
FAQs on Prescription Drug Importation KFF Jordan Medical Devices Importation Regulations Web the food and drug administration (jfda), under the ministry of health (moh), regulates all medical devices in jordan. Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. Web medical device regulations and classification in jordan. The directorate of controlled mds and cosmetics controls medical devices, including. Jordan requires usfda, ce. Jordan Medical Devices Importation Regulations.
From slideplayer.com
Animal Health Status in Jordan All Animal Diseases are obligatory Jordan Medical Devices Importation Regulations The ministry of health (moh). Jordan requires usfda, ce mark, or japanese. Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. The directorate of controlled mds and cosmetics controls medical devices, including. Web medical devices sector in jordan: What certification is required to import medical devices to jordan? Web. Jordan Medical Devices Importation Regulations.
From www.researchgate.net
(PDF) Supply chain practices and organizational performance Evidence Jordan Medical Devices Importation Regulations Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. What certification is required to import medical devices to jordan? Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. The ministry of health (moh). Web staff of directorate of medical devices. Jordan Medical Devices Importation Regulations.
From www.medical-hub.com
Home Page Medical Hub Jordan Medical Devices Importation Regulations The ministry of health (moh). Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. The directorate of controlled mds and cosmetics controls medical devices, including. Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web the food and drug administration. Jordan Medical Devices Importation Regulations.
From www.slideshare.net
Canada medical device approval chart EMERGO Jordan Medical Devices Importation Regulations Web the food and drug administration (jfda), under the ministry of health (moh), regulates all medical devices in jordan. What certification is required to import medical devices to jordan? Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web medical devices sector in jordan: Web a product owner of. Jordan Medical Devices Importation Regulations.
From ib-lenhardt.com
Type Approval for Market Access in Jordan Jordan Medical Devices Importation Regulations What certification is required to import medical devices to jordan? Web medical device regulations and classification in jordan. Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. The ministry of health (moh). The directorate of controlled mds and cosmetics controls medical devices, including. Web staff of directorate of medical devices and. Jordan Medical Devices Importation Regulations.
From www.nhra.bh
National Health Regulatory Authority Bahrain Jordan Medical Devices Importation Regulations The directorate of controlled mds and cosmetics controls medical devices, including. What certification is required to import medical devices to jordan? The ministry of health (moh). Web staff of directorate of medical devices and supplies/cosmetics registration depart Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web a product. Jordan Medical Devices Importation Regulations.
From www.globaldata.com
Jordan Healthcare (Pharma and Medical Devices) Market Analysis Jordan Medical Devices Importation Regulations Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web the food and drug administration (jfda), under the ministry of health (moh), regulates all medical devices in jordan. Web medical devices. Jordan Medical Devices Importation Regulations.
From slideplayer.com
By Hatim Jaber MD MPH JBCM PhD ppt download Jordan Medical Devices Importation Regulations What certification is required to import medical devices to jordan? Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Jordan requires usfda, ce mark, or japanese. Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. The directorate of controlled mds. Jordan Medical Devices Importation Regulations.
From aldar-jordan.com
Homepage AlDar Jordan Medical Devices Importation Regulations Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. Web staff of directorate of medical devices and supplies/cosmetics registration depart What certification is required to import medical devices to jordan? Web medical device regulations and classification in jordan. The directorate of controlled mds and cosmetics controls medical devices, including. Web the. Jordan Medical Devices Importation Regulations.
From medicaldevices.freyrsolutions.com
Medical device registration Japan, PMDA, MHLW, PMDA Medical Device Jordan Medical Devices Importation Regulations Web medical device regulations and classification in jordan. Web staff of directorate of medical devices and supplies/cosmetics registration depart The ministry of health (moh). Web medical devices sector in jordan: Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. What certification is required to import medical devices to jordan? Web the. Jordan Medical Devices Importation Regulations.
From trainunity.net
التسجيل الدوائي و الشؤون التنظيمية, Medical Device Regulations and Jordan Medical Devices Importation Regulations The ministry of health (moh). Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. Jordan requires usfda, ce mark, or japanese. Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web medical device regulations and classification in jordan. The directorate. Jordan Medical Devices Importation Regulations.
From www.slideshare.net
An overview to jordan medical services Jordan Medical Devices Importation Regulations Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web medical devices sector in jordan: Web medical device regulations and classification in jordan. Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. The directorate of controlled mds and cosmetics controls. Jordan Medical Devices Importation Regulations.
From mobilityforesights.com
Jordan Cardiovascular Medical Devices Market 20232030 May 2024 Updated Jordan Medical Devices Importation Regulations Web medical devices sector in jordan: Web medical device regulations and classification in jordan. Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. The ministry of health (moh). The directorate of controlled mds and cosmetics controls medical devices, including. Web staff of directorate of medical devices and supplies/cosmetics registration. Jordan Medical Devices Importation Regulations.
From dohapharma.com
Importation procedure Dohapharma Jordan Medical Devices Importation Regulations Web staff of directorate of medical devices and supplies/cosmetics registration depart The directorate of controlled mds and cosmetics controls medical devices, including. What certification is required to import medical devices to jordan? The ministry of health (moh). Web the food and drug administration (jfda), under the ministry of health (moh), regulates all medical devices in jordan. Web medical device regulations. Jordan Medical Devices Importation Regulations.
From www.facebook.com
Jordan Medical Services Amman Jordan Medical Devices Importation Regulations Web staff of directorate of medical devices and supplies/cosmetics registration depart Web the regulation of medical products according to the principles of good governance and good regulatory practice (1) must also. Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. The directorate of controlled mds and cosmetics controls medical devices, including.. Jordan Medical Devices Importation Regulations.
From www.complete-chain.com
Instructions Complete Chain Doctors Jordan Medical Devices Importation Regulations The directorate of controlled mds and cosmetics controls medical devices, including. Web staff of directorate of medical devices and supplies/cosmetics registration depart What certification is required to import medical devices to jordan? Web a product owner of a medical device system may incorporate medical device and/or accessories from other product. Web the food and drug administration (jfda), under the ministry. Jordan Medical Devices Importation Regulations.